WASTE CONTAINING BOUND NITROGEN
Any burner will form "Thermal NOx" as nitrogen in the combustion air or a waste gas stream reacts with oxygen in the combustion air at flame temperatures. Special Low NOx burners, which control how the fuel or air is added, can minimize the formation of NOx when burning fuel oil or fuel gas. They work by keeping localized flame temperatures low or by keeping local flame excess oxygen low. Thermal NOx formation is limited if the gas temperature is below about 2200oF or if very little excess oxygen is present. Molecular nitrogen (N2) in a waste gas stream acts the same way as N2 in the combustion air stream.
Nitrogen atoms that are bound with other atoms into compounds like ammonia (NH3), nitric acid (HNO3), acetonitrile (C2H3N) or Methyl Amine (CH5N) are much easier to convert to NOx in an incinerator. This is because as the host molecule is oxidized in the furnace or burner, the nitrogen atom is momentarily free to combine with an oxygen atom rather than with another nitrogen atom. Extensive testing has shown that under normal (oxidizing) incinerator conditions, a large percent of any bound nitrogen will convert to NOx. The more dilute the bound nitrogen compounds are in the waste stream, the greater will be the conversion to NOx. Thus, if a waste containing 0.5% amine compounds is incinerated normally, at least 75% of the amine nitrogen will end exit the furnace as NOx. Since nitric acid already contains nitrogen combined with oxygen, almost all of it will convert directly to NOx, regardless of the concentration of the acid in the waste stream.
1. If the waste contains nitrogen gas (N2) no special treatment is needed.
2. If the waste contains compounds that incorporate nitrogen (NH3 ‑ammonia, HCN ‑ hydrogen cyanide, CH5N ‑ amines, etc.) then some fraction of this "bound nitrogen" will combine with oxygen during combustion to form NOx. From 10% to 80% of the bound nitrogen will leave the furnace as NOx (depending on the compound being oxidized and the concentration of the compound in the waste stream) if a normal incinerator is used to dispose of the waste.
3. If the expected amount of NOx leaving the furnace is higher than allowed, a special design is required.
A. A "low NOx" burner design may be all that is needed if bound nitrogen content is low and emission permit levels are high.
B. A multi‑stage furnace may be needed if bound nitrogen or NOx gas in the waste would cause emissions above permit levels. This works by feeding the waste to a high temperature "reducing" furnace, where the nitrogen bearing compounds are broken down, but not enough oxygen is present to combine with the freed nitrogen atoms. The flue gas from the reducing furnace will contain hydrogen and carbon monoxide, but no NOx. This gas is then fed to an "oxidizing" furnace, where the H2 and CO are burned arid only a small amount of thermal NOx is produced.
4. Alternate methods are available are available for removing NOx once it is formed. These include NOx reduction by catalyst (SCR), thermal DeNOx using ammonia injection (SNCR) and various wet scrubber methods. Each of these is expensive, and designing to avoid the NOx formation in the first place is usually preferred.
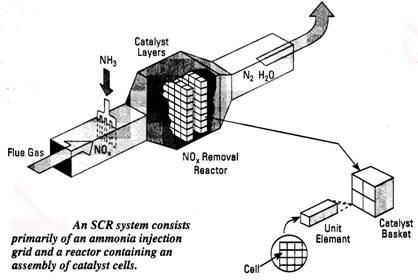
A. Selective Catalytic Reduction (SCR) systems are based on the fact that ammonia will react with NOx molecules to form N2 and H2O when passed over a special catalyst at about 600-700oF. An SCR system includes the ammonia injection system (tank, metering hardware and injection grid to evenly distribute the ammonia in the flue gas stream) and the catalyst vessel. One mol of ammonia is injected for every mol of NOx removed. NOx reduction of up to 90% can be achieved. Part of the ammonia is not reacted, so the product flue gas may contain up to 10 ppm of ammonia slip. Problems include the hardware cost and possible fouling of the catalyst if the flue gas contains particulates. Ammonia injection rate is controlled by a NOx analyzer located downstream of the catalyst vessel as product NOx levels vary, the ammonia rate is adjusted to compensate. Catalyst bed pressure drop is 2-3 water column.

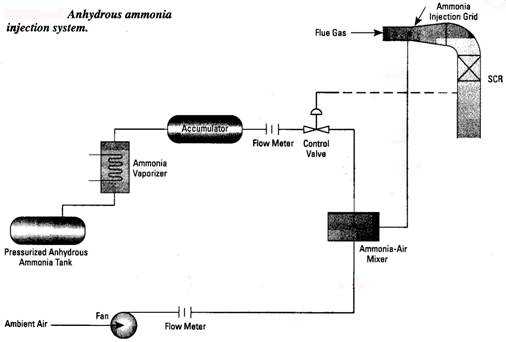
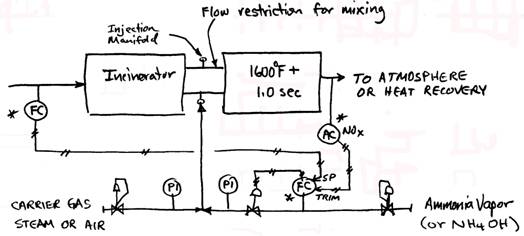
B. Selective Non Catalytic Reduction (SNCR) systems are based on the fact that ammonia will react with NOx molecules at elevated temperatures. The optimum temperature range is 1600 1800oF. Since this is a chemical reaction, about 1 second of reaction time is necessary to get the best NOx removal. Depending on conditions, from 50% of 85% of the NOx in the flue gas can be removed in this way. One mol of ammonia is injected for every mol of NOx removed. Ammonia slip of 10-15 ppm can be expected. An SNCR system includes the ammonia injection system, a refractory lined retention chamber and instrumentation. Ammonia injection rate is controlled by downstream NOx analyzer. The forms of ammonia used include anhydrous (NH3 stored as a liquid and evaporated into the system as needed), aqueous ammonia (ammonium hydroxide NH4OH) and urea (CO(NH2)2). The latter forms are handled as a liquid, while anhydrous ammonia becomes a gas when removed from the storage tank. System pressure drop is about 2 water column.
C. Wet scrubbing systems are relatively complex and expensive. NOx from a combustion process consists of a mixture of about 90% NO and 10% NO2. The NO2 is water soluble and will react with caustic, but most of the NO would pass through a caustic scrubber. In a wet scrubbing process, the combustion flue gas is first quenched with water and then contacted with a chemical which oxidizes the NO to NO2. This gas is then contacted with a caustic solution in a second contactor, where NO2 is removed, forming sodium nitrate. Additional processing may be necessary to eliminate odors from the first contactor.